July 16, 2022 - Onkos Surgical, a privately held specialty medical technology company, today announced that the company's My3D personalized pelvic reconstruction system, has received 510(k) clearance from the FDA for future widespread use in patients addressing complex treatments related to the pelvis.
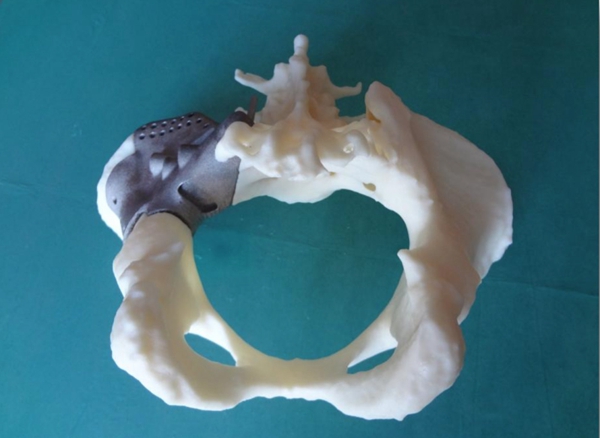
△Repair of pelvis damaged by cancer using 3D printed prosthesis
Onkos Surgical is a leading innovator of fast-growing musculoskeletal oncology, and complex orthopaedic surgery solutions with the My3D Personalised Pelvic Reconstruction System, the first solution of its kind to include 3D printed implants, instruments and models, as well as a comprehensive platform for the treatment of deformities, trauma, disease and other treatments, or for advanced planning to address surgical failure remodelling services.
The products include patient-specific implants, as well as advanced reconstruction for acetabular reconstruction and multiple regions across the pelvis. The implants are designed and printed with unique features to help address the challenges of bone and soft tissue attachment, as well as anatomical repair accuracy. This PFA approval will once again expand the company's portfolio of oncology, and complex orthopaedic solutions.
Matthew Seidel, a physician with HonorHealth Orthopaedics in Scottsdale, Arizona, said. " This service offered by Onkos Surgical will greatly advance the way my colleagues and I treat these patients," "Patients with these pelvic conditions face many clinical challenges. Historically, our implant options were mass-produced and perhaps they were not for every patient. Now, Onkos offers us a new option. By allowing us to plan ahead with virtual planning" for surgery and deliver patient-specific implants and devices within a few weeks, it has changed the way I treat patients."
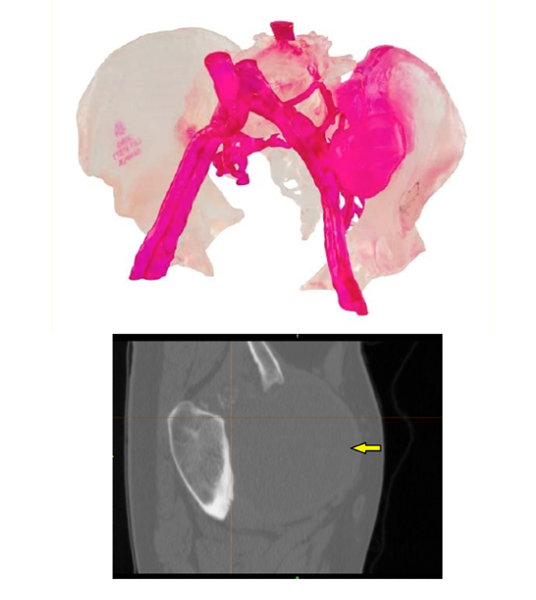
△The company uses My3D technology to develop personalised implant, device and anatomical model designs to help surgeons better perform surgical procedures
My3D and the digital ecosystem
The My3D service includes access to the company's digital ecosystem, where surgeons can upload medical images of patients in an encrypted, HIPAA-compliant online format, and the platform then renders the anatomical images into 3D models for surgeons to collaborate on virtual surgery planning sessions to remove diseased bones and precisely match patients' implants. With this license and capability, Onkos can meet surgical requirements from start to finish within six weeks. Patrick Treacy, co-founder and CEO of Onkos Surgical, said, "This approval is an important milestone for our company as we continue to leverage the expertise and experience we have gained in 3D printing to continue to optimise the technology for specific solutions to complex orthopaedic conditions. " "We strongly believe that patients suffering from these conditions deserve solutions specifically designed for them. Our My3D platform and digital ecosystem not only allows us to deliver personalised solutions faster, but also sets the stage for the future of musculoskeletal personalisation."
It is reported that over 350 leading healthcare providers in the US, have chosen Onkos' innovative 3D printed orthopaedic solution. This approach leverages the latest innovations in virtual surgical planning, 3D anatomical modelling and printing, implant design and workflow optimisation to support improved patient outcomes and experience.
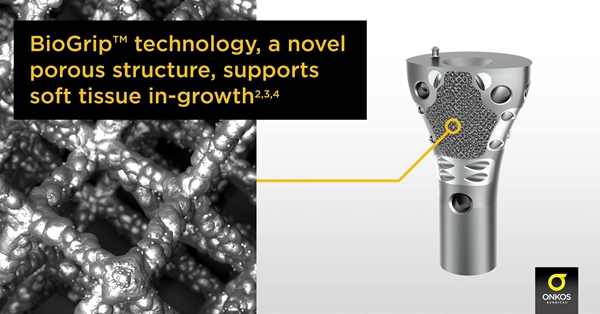
△ Onkos Surgical's BioGrip 3D printed collar
In 2017, the company received 510(k) clearance from the FDA for its 3D printed collar implant, which is available as a modular collar for musculoskeletal oncology and complex orthopaedic limb preservation procedures, as well. These devices use 3D printing technology tailored to each patient to improve bone perfusion and, according to the company, reduce the clinical challenges associated with loosening of orthopaedic limb implants.
Limb preservation surgery, a procedure that extracts a tumour from an extremity without removing the entire limb. Even if a small amount of cancer is left behind, it can spread to other parts of the body. As a result, the surrounding bone and tissue is usually also removed, in which case implants can be used to restore the appearance and function of the limb.

ΔThe market size and share of 3D printed medical implants is set to achieve a lucrative CAGR
Trends in 3D printed implants
The 3D printed implant industry has been on the rise with great success for some time now. This includes the treatment of patients with spinal problems that require implants. Previously, patients had to receive an already manufactured implant, but with 3D printing, patients can now receive implants that are customised to their individual anatomy, which not only reduces discomfort and revision surgery, but also prevents paralysis and death. Another such case is the study of jaw implants, where there have been problems with patient rejection in the past and 3D printing can help by providing patient-specific solutions.
However, the FDA has encountered challenges in trying to regulate, monitor and ensure the safety of medical devices produced by 3D printing. The technology now allows for a decentralised, highly customisable form of manufacturing, often by organisations with limited experience of FDA guidelines. Since these medical products may be created at the point of care and the FDA has no jurisdiction over medical practices, the distinction between product and practice can be difficult to discern and regulate.
Despite this, Scott Dunham, vice president of research at SmartTech, estimates the 3D printed healthcare industry to be worth $6.08 billion by 2027, compared to $1.25 billion in 2019. an estimated 600,000 implants were produced in 2019, of which the number is expected to increase to 4 million by 2027.




