3D-printed bone grafts from medical device manufacturer Cerhum have been approved for use in patients throughout Europe. MyBone is reportedly the first commercially available3D-printed bone graft authorized under medical device regulation 2017/745 and is also ISO 13485 certified. As a result, patient-specific bone grafts will now be available to maxillofacial and plastic surgeons across the continent.
Grégory Nolens, Cerhum's founder and CSO, said, "We are very proud to have successfully collected important clinical data and overcome all regulatory hurdles with safe and effective synthetic bone grafts."
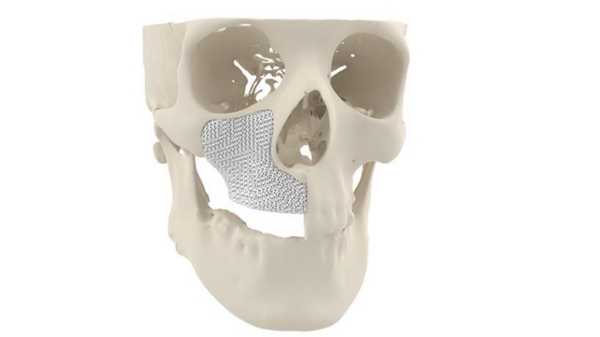
Cerhum's MyBone 3D printed facial bone graft. Image from Cerhum.
3D Printed Maxillofacial Implants
Additive manufacturing is increasingly being used in maxillofacial surgery in the form of guidelines to enhance patient outcomes and, more recently, to create facial grafts that can be implanted in patients' skulls. For example, the Centre de Recherche Industrielle du Québec (CRIQ) is 3D printing patient-specific mandibular implants, while researchers at Paulista University have 3D printed facial prostheses for Brazilian cancer survivors. Meanwhile, earlier this year, Health Canada approved its first Canadian-made 3D printed medical implant for facial reconstruction surgery for oral cancer patients.
In May, a group of Korean researchers conducted a retrospective study to verify the effectiveness and safety of 3D-printed titanium implants in the maxillofacial skeleton. The study looked at the results of 16 patients suffering from various maxillofacial defects, and almost all of the implants showed satisfactory treatment results.
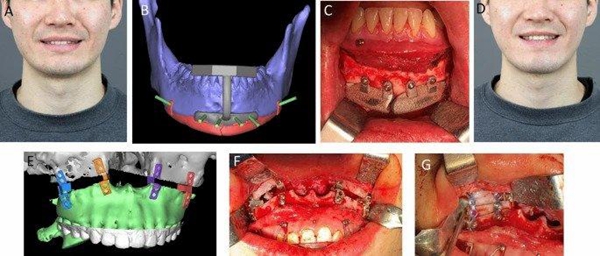
One of the restorative surgery case studies. Image from Nature.
MyBone Bone Graft
Cerhum (Human Ceramics) was founded in 2016 as a spin-off company of Sirris, the Belgian National Institute of Science and Technology. In collaboration with the University of Liege, the company aims to replace autologous grafts - bones taken from other parts of a patient's body - with synthetic 3D-printed replacements. To date, the company has 3D printed tens of thousands of synthetic bone grafts, which have been used by a range of companies for orthopedic implants. myBone is a patient-specific 3D printed bone graft designed to treat patients with severe facial deformities. The graft is made from hydroxyapatite, the primary mineral component of natural bone, and 3D printed with a unique porous structure to promote bone growth.
"Our 3D printed bone implants offer a unique patented porous structure that allows blood vessels to grow inward," Nolens said. "This process, called vascularization, is the key to successful inward bone growth. As a result, MyBone's bone grows inward seven times faster than currently available bone graft particles." MyBone is now validated for use in European patients under Medical Device Regulation 2017/745 and is registered with the Belgian authorities. The bone graft is also ISO 13485 certified, so it is now available for surgeons to use as a patient-specific implant for maxillofacial surgery. One patient received a MyBone graft implant as part of a controlled release phase two years ago, and the implant now reportedly looks identical to natural bone, according to a recent CT scan.
"Given the complexity of the defect, it was aesthetically impossible to achieve such a perfect functional result using existing methods," explained Dr. Christophe Ronsmans, head of CHR Liege's Department of Plastic Surgery. The patient's condition is said to be "excellent" and the success of the procedure has paved the way for the commercial application of MyBone in Europe. "Due to the positive feedback from maxillofacial surgeons so far, Cerhum is expanding its portfolio to the dental and oncology markets," Nolens added.



